What is calcium carbonate?
Table of Contents
Description of calcium carbonate
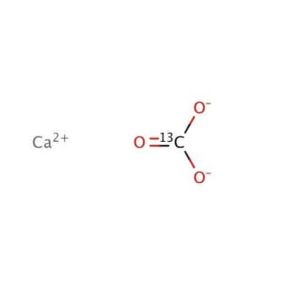
Calcium carbonate is an inorganic compound CaCO₃. It is the main ingredient in limestone, marble, etc. Calcium carbonate is basically insoluble in water but soluble in hydrochloric acid. It is one of the common substances on earth, found in aragonite, calcite, chalk, limestone, marble, travertine and other rocks, and is also the main component of the bones or shells of some animals. Calcium carbonate is also an important building material with a wide range of industrial uses.
Physical properties
Calcium carbonate is a white fine crystalline powder, tasteless and odorless. There are two forms, amorphous and crystalline. The crystal types can be divided into rhombic and hexagonal crystal systems, which is columnar or rhomboid with a density of 2.93g/cm3. The melting point is 1339℃ (decomposed at 825-896.6℃), and 1289℃ at 10.7MPa. Insoluble in alcohol, soluble in ammonium chloride solution, almost insoluble in water.
Chemical properties
1.Calcium carbonate is decomposed into calcium oxide and carbon dioxide at 825-896.6℃.
CaCo3 = CaO + CO2 ↑
2.Calcium carbonate will bubble and boil with dilute acid (such as dilute acetic acid, dilute hydrochloric acid, dilute nitric acid, etc.) and dissolve. The reaction also emits carbon dioxide, an exothermic reaction. For example, it reacts with dilute hydrochloric acid to form calcium chloride, water and carbon dioxide.
CaCo3 + 2HCl= CaCl2 + H2O+ CO2 ↑
3.Water mixed with CaCO3 passes through excess carbon dioxide to form a solution of calcium bicarbonate. Calcium carbonate reacts with carbonic acid solution to form calcium bicarbonate. CO2 was added to the turbid limestone water and the precipitation disappeared.
CaCo3 + H2CO3 = Ca(HCO3)2
Or CaCo3 + CO2 + H2O= Ca(HCO3)2
4.Anhydrous calcium carbonate is converted into calcite by heating to 1000K.
Application of calcium carbonate


Calcium carbonate is used in the paint and waterborne coating industry.
Calcium carbonate is used in the plastics and paper industry.
Calcium carbonate is used in feed and fertilizer industries.
Calcium carbonate powder is used for flue gas desulfurization in coal-fired power plants.
Calcium carbonate is used as a filler in the rubber industry.
Calcium carbonate is used in feed and fertilizer industries.